> Definition of solids
Solids are collection of molecules with strong intermolecular force, overcoming this force causes a phase transition.
> charges
Showcase of vander wall’s force of attraction.
> wall
Opening the wall, it forms molecule of H2, but as of now it’s single Hydrogen atom.
> new object
Hydrogen Molecule with a shared e- between them.
> Total interactions,
Since there are 5 electrons and 4 protons, interaction is a combination of these,
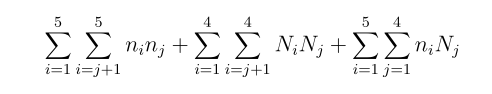
n is for the electrons, N is for the protons.
> “d”
Smallest possible d so, the total energy term is more negative.